Nanocavity-modified Ground State Chemistry
Articles: published in Angewandte Chemie and Physical Review X by Javier Galego, Clàudia Climent Biescas, Johannes Feist and Francisco J. García-Vidal, IFIMAC researchers and members of Department of Theoretical Condensed Matter Physics.
Over the past few years it has become clear that strong electromagnetic coupling between a molecule and light confined in a nanoscale cavity can lead to significant modifications of the electromagnetic response of the hybrid system as well as the internal molecular properties. This has already been exploited to manipulate the fate of photochemical reactions, however, up to now there has been no general theory on how these interactions affect the chemical reactivity of a molecule in its ground state without any external input of energy.
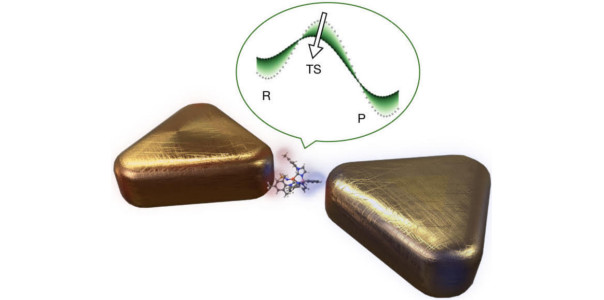
In a theoretical study published in Physical Review X, a group of researchers from the Departamento de Física Teórica de la Materia Condensada and the Condensed Matter Physics Center (IFIMAC) at the Universidad Autónoma de Madrid have developed a theoretical framework that combines quantum electrodynamics and the quantum theory of chemical reactivity. The authors implemented this approach for a simple model molecule that can be solved without approximations. This allowed to explore the general properties of cavity-induced ground-state chemical reactivity changes and develop a simplified theoretical model that can be applied to more complex molecules. They found that the induced changes on the molecular pontential energy surfaces do not depend on any resonance condition between molecular transitions (such as vibrational or electronic excitations) and cavity modes, with the relevant interactions closely related to well-known electrostatic forces on the molecule due to other molecules or larger material bodies. In particular, the largest contribution to the modification of the energy landscape is typically determined by the change of the permanent dipole moment of the molecule between its equilibrium and transition state configuration. They have also shown that experimentally available nanocavities can induce changes in reaction rates by an order of magnitude in a single molecule, while for the case of many molecules, this effect becomes significant only if all the molecules are aligned.
In a second paper published in Angewandte Chemie, the same authors have combined this theory with quantum chemistry calculations and have shown how plasmonic nanocavities can enable self-induced electrostatic catalysis of typical organic reactions, without any external driving, due to the interaction of the molecular permanent and fluctuating dipole moments with the plasmonic cavity modes. They have also exploited this interaction between molecules and electromagnetic modes to predict cavity-induced changes in the transition temperature of spin-crossover transition metal complexes, the prime example of molecular switches.
These studies contribute to the fundamental understanding of how nanoscale cavities can be used to manipulate chemical reactions of single molecules and open the path towards ground-state catalysis with plasmonic nanocavities. They also provide an example on how the interaction with plasmonic modes may be ultimately exploited to manipulate material properties. [Angewandte Chemie – full article] [Physical Review X – full article]