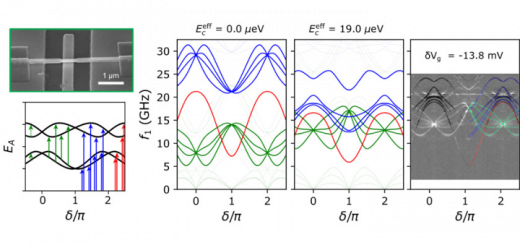Cavity-Induced Modifications of Molecular Structure in the Strong-Coupling Regime
 Article: published in Physical Review X by Javier Galego, Francisco J. Garcia-Vidal, and Johannes Feist, Department of Theoretical Condensed Matter Physics and IFIMAC researchers.
Article: published in Physical Review X by Javier Galego, Francisco J. Garcia-Vidal, and Johannes Feist, Department of Theoretical Condensed Matter Physics and IFIMAC researchers.
When organic molecules interact with light modes confined in nanostructures, hybrid light-matter states (polaritons) can be created via so-called “strong coupling.” The participating molecules are affected by the interaction, and this effect can be used for controlling chemical reactions or modifying a material’s properties. Up until now, there has been no consensus on how these modifications occur, and there has accordingly been no way to accurately predict these modifications or design novel structures to exploit them. Now, IFIMAC researchers Javier Galego, Prof. Francisco J. García Vidal, and Johannes Feist demonstrated that it is possible to understand and predict these modifications by adapting well-known techniques from chemical physics. The extent of the modifications turns out to depend sensitively on which observable is interrogated.
Progress up until now had been hindered by the fact that organic molecules possess a large number of internal (rovibrational) degrees of freedom and do not behave like simple two-level emitters. The researchers employed a first-principles approach that takes into account electronic, nuclear, and electromagnetic degrees of freedom on an equal footing. They demonstrated that the Born-Oppenheimer approximation, which underlies most of our understanding of chemical structure and dynamics and assumes that nuclear and electronic motion can be separated, can break down under strong coupling. However, exactly how this happens can be predicted, and in addition, in many important cases it does not break down. By exploiting this approach, the researchers show that molecular structure is modified even for so-called dark states, which are typically thought to be uncoupled from electromagnetic modes. These findings provide the basis for understanding how to manipulate chemical structure and reactions through strong coupling, and could have wide-ranging implications for the design of novel nanostructures. [Full article]