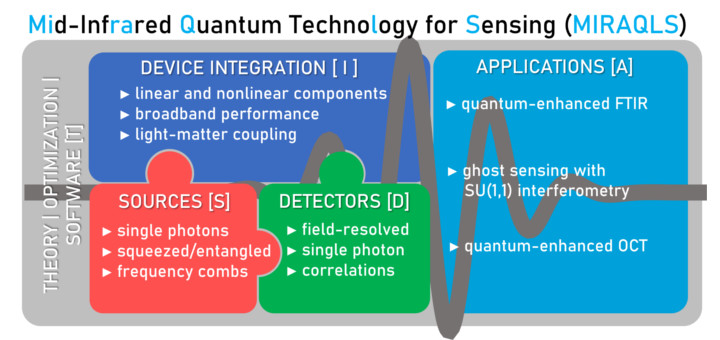
Joint EU/Canada Project MIRAQLS Coordinated by IFIMAC Researchers Starts
The European Union and Canada just announced support for three jointly funded projects in quantum research and innovation: https://digital-strategy.ec.europa.eu/en/news/joint-eucanada-quantum-research-projects. We are happy to announce that one of those three projects is MIRAQLS on Mid-Infrared Quantum...