Resonant Tunneling in Protein-based Junctions
Article: published in PNAS by Juan Carlos Cuevas, IFIMAC researcher and member of the Department of Theoretical Condensed Matter Physics.
Proteins play a fundamental role in numerous biological energy conversion processes such as photosynthesis, respiration, and a wide variety of enzymatic reactions. In recent years, redox proteins containing transition metal ion centers have been integrated into solid-state electronic junctions. The goal is to shed new light on the electron transfer mechanisms in these biomolecules, but also to investigate the possibility of using proteins as active elements in novel, bio-inspired electronic devices. In this context, recent experiments have shown that the electron transport through proteins can be surprisingly efficient. However, the origin of this efficiency and, in general, the underlying transport mechanisms remain largely unknown.
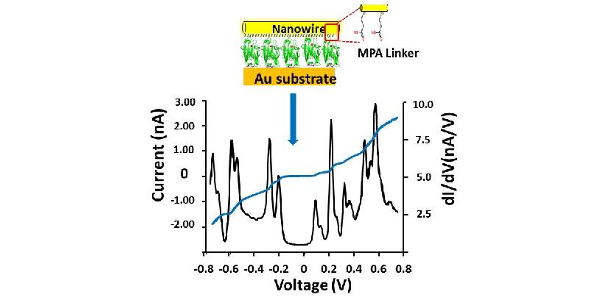
New light on this fundamental problem has now been shed in a work published in the Proceedings of the National Academy of Sciences of USA (PNAS) by a collaboration between the group of David Cahen (Weizmann Institute of Science, Rehovot, Israel) and the IFIMAC researcher Juan Carlos Cuevas. In this work, these researchers report low-temperature (10 K) electron transport measurements via monolayer junction based on the blue copper protein Azurin that strongly suggest that quantum tunneling is the dominant charge transport mechanism. In particular, they show that weakening the protein-electrode coupling by introducing a spacer, one can switch the electron transport from off-resonant to resonant tunneling, which has never been reported before in protein-based junctions. Moreover, vibronic features of the Cu(II) coordination sphere were identified in the transport characteristics, which shows directly the active role of the metal ion in the resonant tunneling. These results illustrate how quantum mechanical effects may dominate electron transport via protein-based junctions, which is clearly at variance with the common wisdom in the field of protein electron transfer in biological settings. [Full article]